The TOOLMED Titanium PLIF Lumbar Cage is a high-strength interbody fusion device engineered for Posterior Lumbar Interbody Fusion (PLIF) procedures. Constructed from medical-grade Titanium Alloy (Ti6Al4V), this cage is designed for surgeons who prioritize the superior primary stability and excellent biocompatibility of metallic implants. The cage features a roughened surface texture to enhance initial friction and promote rapid osseointegration between the vertebral endplates and the implant. Designed to be inserted in pairs via a posterior approach, the Titanium PLIF Cage provides exceptional structural support to the anterior column, effectively restoring disc height and sagittal alignment. Its large internal chamber allows for substantial bone graft volume, while the inherent radiopacity of titanium provides unmistakable visualization under fluoroscopy. With its aggressive tooth pattern and anatomical biconvex shape, the TOOLMED Titanium PLIF Cage is a reliable solution for achieving robust spinal fusion in patients with lumbar instability.
Spine
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
The specialized titanium surface encourages direct bone apposition, allowing for a more biological interface between the implant and the vertebral endplates compared to traditional polymers.
Forged from medical-grade Ti6Al4V, this cage is ideal for high-load applications in the lumbar spine, providing rigid structural support even in cases with significant vertebral instability.
The superior and inferior surfaces are equipped with sharp, unidirectional teeth that bite into the subchondral bone, providing exceptional primary stability and preventing postoperative migration.
Despite its high-strength metal construction, the cage features a maximized internal chamber to ensure a large volume of bone graft can be packed, facilitating a solid bone bridge across the disc space.
Indicated for weight-bearing support in patients with symptomatic DDD. The titanium construction is particularly effective for restoring height in severely collapsed levels where high-strength distraction is required.
Used to stabilize spinal segments that have slipped forward. The rigid titanium interface provides the shear resistance necessary to stop slip progression and maintain corrected sagittal alignment.
Specifically indicated for indirect decompression. By distractively restoring disc height, the titanium cage effectively re-opens the neural foramen and spinal canal, alleviating nerve root compression.
While used with caution, the increased surface area of our Titanium PLIF design is applied to provide a stable foundation in patients where higher mechanical strength is needed to support the anterior column.

| Product name | Titanium PLIF Lumbar Cage |
| Material | TA3 |
| Diameter | / |
| Length | / |
| Application | / |
| Certificate | CE Certificate |
| Brand | TOOLMED |
| MOQ | 1 Pcs |
| OEM | Avaliable |
| Package | PE Inner Bag+Carton |
| Payment Method | T/T,Bank transfer, Western Union |
| Delivery Time | 5-7 days |
| Shipping | DHL EMS UPS TNT FEDEX |


The Titanium PLIF Lumbar Cage is a metallic interbody fusion device designed for use in posterior lumbar spinal reconstruction. Unlike polymer-based spacers, this cage is manufactured from biocompatible Titanium Alloy (Ti6Al4V), which is prized for its high mechanical strength and its ability to integrate directly with bone tissue. It acts as a structural bridge, replacing the intervertebral disc and providing the necessary height and stability to facilitate permanent bone fusion between adjacent lumbar vertebrae.
This cage is applied primarily in bilateral Posterior Lumbar Interbody Fusion (PLIF) procedures. It is used to treat patients suffering from advanced degenerative disc disease, segmental instability, and spondylolisthesis. Because of its rigid nature, it is frequently chosen for cases where high resistance to axial compression is required or where a metal-on-bone interface is preferred to stimulate osteoblastic activity during the fusion process.
The primary advantage of the titanium cage is its superior osseointegration potential. The surface of the titanium alloy promotes the attachment and growth of bone cells directly onto the implant. Additionally, its high fatigue strength ensures that the disc height is maintained even under heavy physiological loads. The inherent radiopacity of the material allows for absolute certainty in verifying the cage's position during postoperative imaging.
Biomechanically, the Titanium PLIF cage provides rigid anterior column support. Its anatomical biconvex design ensures a wide distribution of pressure across the endplates, while the aggressive unidirectional teeth provide high shear resistance. This mechanical locking mechanism, combined with the high friction coefficient of titanium, ensures that the segment remains stable during patient movement, protecting the developing bone graft bridge.
Following a posterior approach and bilateral discectomy, the vertebral endplates are prepared to bleeding bone. A trial cage is used to select the correct size. The Titanium PLIF Cage is then filled with autograft or bone substitute and inserted into the disc space. Due to its rigid nature, precision in insertion is key. Final positioning is confirmed via X-ray, showing the clear metallic outline of the cage, and the construct is usually reinforced with posterior pedicle screws.
Postoperative care focuses on gradual rehabilitation and monitoring the metal-bone interface. Patients may wear a lumbar support for several weeks to limit high-impact activities while the titanium surface undergoes osseointegration. Follow-up X-rays are used to confirm that the cage remains perfectly seated. While titanium can cause some "bloom" on CT scans, modern imaging protocols allow for clear assessment of the fusion mass surrounding the implant.
The TOOLMED Titanium PLIF Lumbar Cage offers an exceptionally stable and biologically compatible solution for lumbar fusion. Its metal construction provides the mechanical durability and osseointegration properties necessary for successful outcomes in complex spinal reconstructions.
Titanium offers better initial osseointegration and surface bone growth, while PEEK offers radiolucency. Many surgeons prefer titanium for its rigid strength and proven biological performance in metal-on-bone contact.
Titanium is radiopaque, meaning it is clearly visible on X-rays. While it produces more artifact on CT/MRI than PEEK, its position can be verified with 100% accuracy on standard fluoroscopy.
The biconvex design matches the natural dip in the center of the vertebral endplates, providing a better anatomical fit and reducing the risk of the cage shifting after surgery.
For a PLIF procedure, these cages are almost always used in pairs—one on the left and one on the right—to provide balanced, bilateral support for the lumbar segment.
Yes, the large internal window is designed to be packed with various graft materials, including autograft, allograft, or synthetic bone substitutes like BMP.
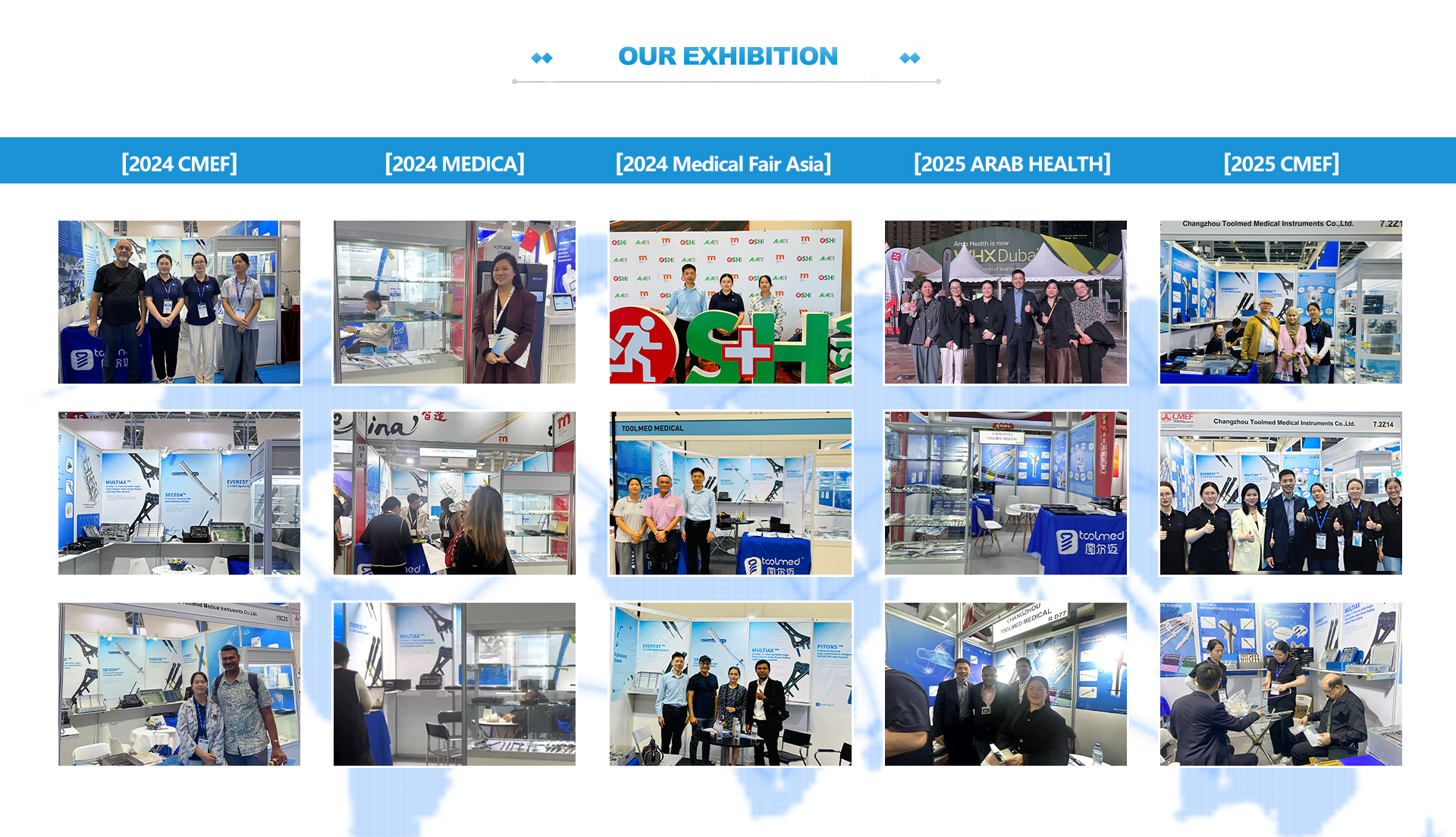

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us