Spine
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
The Series II features a modified footprint that maximizes the contact area with the vertebral endplates, distributing axial loads more efficiently and providing a stable foundation for fusion.
The internal window has been maximized to accommodate a larger volume of bone graft or bone substitute, increasing the biological potential for a solid, multi-level fusion bridge.
Featuring an upgraded tantalum marker configuration, the Cage-II allows for easier assessment of both depth and tilt under fluoroscopy, ensuring perfect anatomical alignment.
The refined tooth geometry on the superior and inferior surfaces is designed to bite into the subchondral bone without causing excessive trauma, ensuring the cage remains locked in position.
The Cage-II is specifically indicated for maintaining disc height after the removal of a herniated cervical disc, preventing the collapse of the intervertebral space and subsequent nerve irritation.
It is used to treat chronic instability caused by age-related wear in the neck. The cage stabilizes the motion segment, reducing the mechanical pain associated with osteophyte formation and facet joint stress.
Indicated for patients presenting with arm pain or weakness (radiculopathy) due to foraminal narrowing. The cage re-opens the neural foramen through height restoration, providing immediate relief for compressed nerves.
The Series II is an excellent choice for revision cases where a previous fusion attempt has failed. Its increased surface area and graft volume help overcome the biological challenges of achieving a secondary fusion.

| Product name | Cervical Peek Cage-II |
| Material | TA3 |
| Diameter | / |
| Length | / |
| Application | / |
| Certificate | CE Certificate |
| Brand | TOOLMED |
| MOQ | 1 Pcs |
| OEM | Avaliable |
| Package | PE Inner Bag+Carton |
| Payment Method | T/T,Bank transfer, Western Union |
| Delivery Time | 5-7 days |
| Shipping | DHL EMS UPS TNT FEDEX |

The Cervical Peek Cage-II is a second-generation interbody fusion device specifically designed for the cervical motion segments (C3-C7). It is an implantable spacer made from medical-grade PEEK polymer that replaces the natural disc after surgical removal. The "II" designation emphasizes its refined anatomical geometry, which provides a larger load-bearing surface and optimized graft volume to facilitate a faster and more robust biological fusion process in the neck.
This cage is applied during Anterior Cervical Discectomy and Fusion (ACDF) procedures to treat neurological deficits and chronic pain. It is the preferred choice for multi-level cervical reconstructions where maximum stability and graft containment are essential. The Series II design allows surgeons to treat a wide range of patient anatomies, from narrow to wide disc spaces, ensuring a personalized and secure fit for every patient.
The primary advantage of the Series II is its enhanced stability profile. The larger footprint reduces the pressure exerted on the vertebral endplates, further lowering the risk of subsidence in osteoporotic patients. Additionally, the PEEK material is completely radiolucent, allowing surgeons to visualize the exact quality of the bone fusion inside the cage on follow-up X-rays without the "starburst" artifacts common with metal implants.
Biomechanically, the Cage-II functions as a structural pillar that restores the anterior column's load-bearing capacity. Its biconvex profile ensures it sits flush against the natural contours of the vertebrae, while the mechanical serrations provide high resistance to expulsion. The material's bone-like elasticity ensures that the bone graft within the cage is physiologically loaded, promoting the natural "Wolf's Law" of bone remodeling and fusion.
After a thorough anterior discectomy, the surgeon prepares the vertebral endplates to a bleeding surface. A dedicated Series II trial is used to determine the exact size and lordotic angle needed. The cage is then packed with bone graft, attached to the Series II inserter, and impacted into the disc space. Tantalum markers are checked via fluoroscopy to ensure symmetrical positioning before closing or applying a supplemental cervical plate.
Patients are typically advised to use a cervical collar for several weeks post-surgery to maintain alignment while the fusion matures. Regular follow-up imaging is scheduled to monitor the incorporation of the bone graft. Because PEEK is non-conductive and non-magnetic, patients can safely undergo MRI or CT scans at any time postoperatively for neurological evaluation without concern for implant interference.
The TOOLMED Cervical Peek Cage-II offers an advanced solution for cervical spinal fusion, combining material excellence with anatomical precision. Its enhanced design ensures maximum stability and fusion success, making it a premier choice for surgeons treating complex cervical degenerative conditions.
The "II" series generally offers an updated footprint and modified internal geometry, allowing for a larger graft area and a more customized fit for diverse patient anatomies compared to the standard Series I.
While the cage provides high primary stability due to its tooth design, most clinical protocols for ACDF recommend using a supplemental anterior cervical plate to ensure maximum safety and fusion rates.
Yes, PEEK-OPTIMA is a high-performance polymer used in millions of spinal implants worldwide. It is specifically chosen for its high fatigue strength and long-term durability in weight-bearing environments.
Because the PEEK cage is invisible on X-rays, the tantalum pins provide visual cues so the surgeon can verify that the cage is centered and at the correct depth within the disc space.
The Cervical Peek Cage-II is designed for the C3 to C7 segments, but depending on the patient's anatomy, it can be used for the C7-T1 junction if the footprint matches the vertebral endplate size.
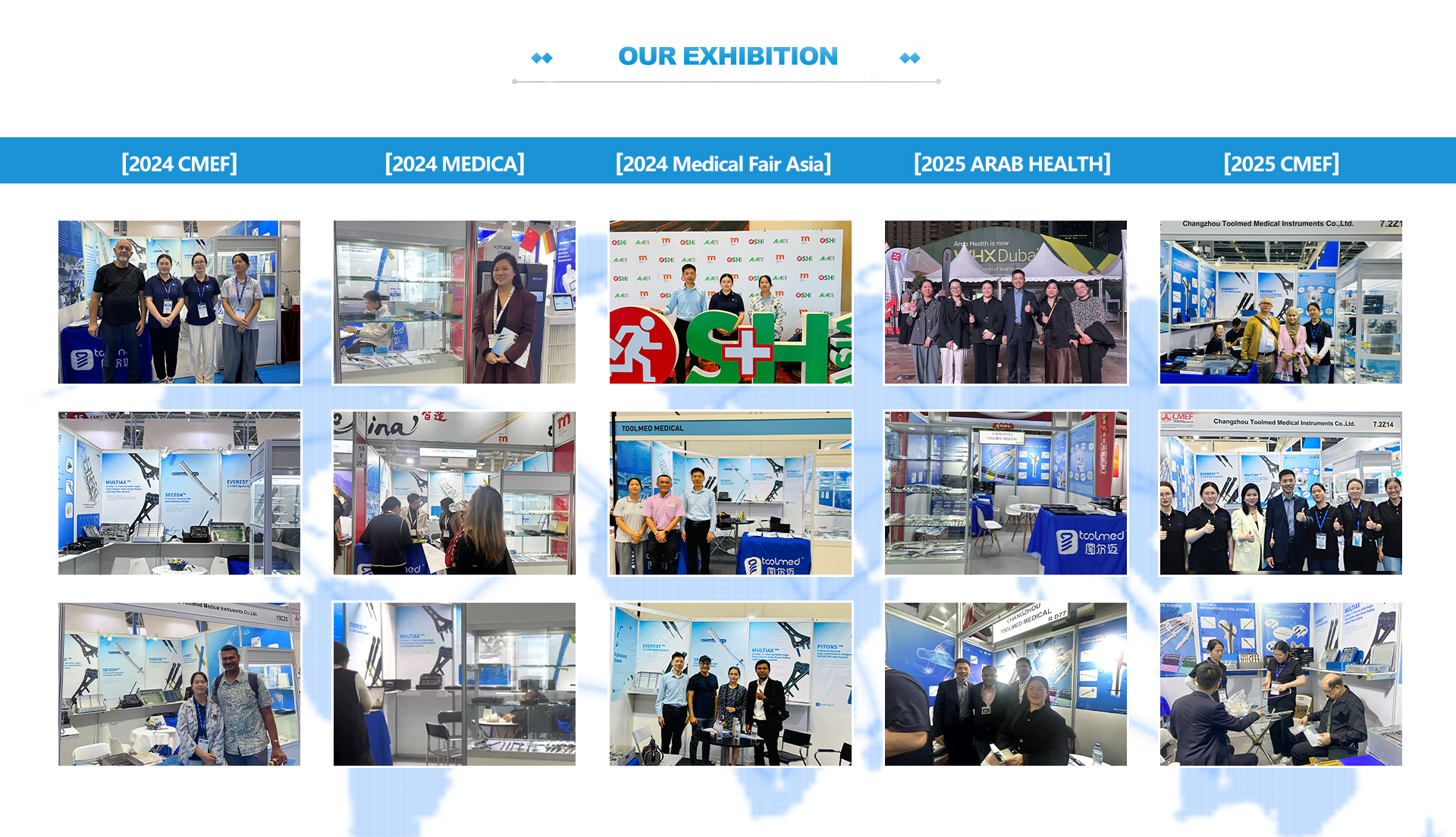

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us