Spine
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
The core advantage of the expandable design is the ability to adjust the cage's height after insertion, allowing for precise restoration of the spinal column's natural alignment and height.
With a low-profile collapsed state, the cage can be inserted through smaller surgical windows, significantly reducing the need for extensive soft tissue and nerve root retraction.
Once the desired height is achieved, the internal self-locking mechanism ensures the cage maintains its position under physiological loads, preventing postoperative subsidence or collapse.
The superior and inferior surfaces feature a textured or spiked "bullet" profile that bites into the subchondral bone, providing immediate primary stability and resisting implant migration.
While primarily utilized in the spine, the core technology of expandable scaffolds is occasionally adapted for massive proximal humerus bone loss in limb-salvage oncology cases, providing a height-adjustable structural column where traditional fixed spacers may fail.
In complex revision hip arthroplasty, the expandable concept is applied to acetabular distractors. These devices help restore the center of rotation and manage segmental defects in the pelvic ring by providing controlled expansion against the ilium and ischium.
Expandable technology serves as a "growing" or adjustable spacer in pediatric orthopedic oncology. It allows for the reconstruction of distal femoral defects, providing a stable, load-bearing bridge that can be adjusted to match the patient's contralateral limb length.
The primary indication is Thoracolumbar Vertebral Body Replacement. It is used to reconstruct the anterior column after a corpectomy for burst fractures, primary or metastatic spinal tumors, and severe infections (osteomyelitis) where a customizable height is required to bridge the defect.

| Product name | Expandable Titanium Cage |
| Material | TA3 |
| Diameter | / |
| Length | / |
| Application | / |
| Certificate | CE Certificate |
| Brand | TOOLMED |
| MOQ | 1 Pcs |
| OEM | Avaliable |
| Package | PE Inner Bag+Carton |
| Payment Method | T/T,Bank transfer, Western Union |
| Delivery Time | 5-7 days |
| Shipping | DHL EMS UPS TNT FEDEX |


The Expandable Titanium Cage is a specialized vertebral body replacement (VBR) device used to reconstruct the spine's anterior column. It consists of a telescoping mechanism that allows the implant to be inserted in a collapsed state and then expanded to the exact required height. This adjustability ensures an optimal "press-fit" between vertebrae, providing immediate mechanical support while facilitating biological fusion through the bone graft packed inside its hollow core.
The primary application is for the treatment of severe spinal instability following a corpectomy (removal of a vertebral body). It is used across the thoracic and lumbar spine to manage burst fractures, stabilize the spine after tumor resection, and reconstruct the anterior column in cases of severe osteomyelitis (bone infection) or kyphotic deformity correction.
The main advantage is the reduction of surgical risk; by inserting the cage in a collapsed state, surgeons can avoid over-distraction of nerve roots. Furthermore, the ability to fine-tune the expansion in situ allows for better restoration of lordosis and sagittal balance compared to traditional fixed-height mesh cages. The self-locking mechanism provides superior resistance against postoperative subsidence.
Biomechanically, the expandable cage serves as a load-sharing pillar that replaces the weight-bearing function of the anterior spinal column. Its design distributes axial forces across the vertebral endplates, reducing peak stress points. The titanium alloy construction offers a modulus of elasticity that balances structural rigidity with the need to stimulate bone growth, minimizing the risk of the "stress shielding" effect.
The procedure begins with a thorough corpectomy and endplate preparation. A trial spacer is used to estimate the required height. The Expandable Titanium Cage is then connected to a specialized inserter-driver instrument and placed into the defect. Using a torque-limited driver, the cage is expanded until it firmly engages the endplates. Once the height is verified via fluoroscopy, the locking screw is tightened, and bone graft is packed around the device.
Postoperative care typically includes early mobilization with the support of a rigid or semi-rigid brace to protect the reconstruction during the initial fusion phase. Follow-up imaging with X-ray or CT is performed at regular intervals to monitor the progress of the bone graft incorporation and ensure the maintenance of the restored spinal height.
The TOOLMED Expandable Titanium Cage represents a significant advancement in spinal reconstruction, offering a customizable, stable, and minimally invasive solution for complex vertebral defects. Its precision expansion and high-strength titanium design provide patients with the best opportunity for a successful fusion and restored quality of life.
No, the TOOLMED system features a high-torque internal locking mechanism that prevents the telescoping cylinders from retracting once the desired height is set and locked.
The cage features large lateral windows and a hollow center. Surgeons typically pack the center of the cage before insertion and add additional bone graft around the device after expansion is complete.
This specific system is designed for the larger loads of the thoracic and lumbar spine. For the neck, we offer smaller Expandable Cervical Cages specifically tailored for cervical anatomy.
Yes, the TOOLMED Expandable Cage must be used with its dedicated instrument set, which includes the inserter, expansion driver, and torque-limited handles to ensure safe deployment.
Our largest model can expand up to 85mm, making it suitable for multi-level corpectomies where a significant portion of the spinal column must be replaced.
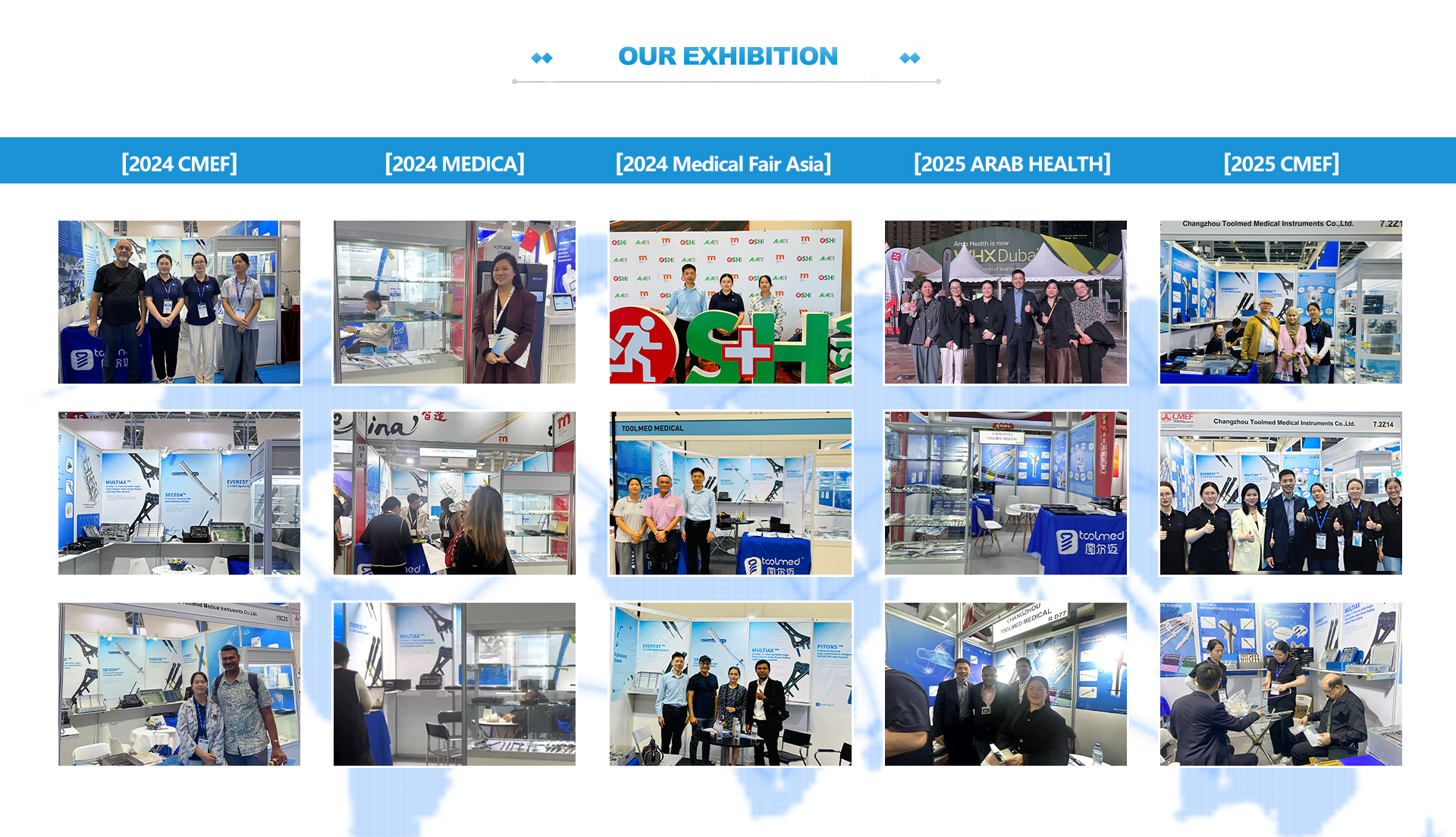

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us