Constructed from medical-grade PEEK (Polyether Ether Ketone), the cage incorporates titanium alloy marker pins to enhance intraoperative visualization. Available in multiple footprints, heights (e.g. 7–17 mm), and lordotic angles (0° or 5°), T‑PAL is optimized for anatomical alignment and effective intervertebral fusion. CE- and ISO-certified, it's indicated for lumbar posterior or transforaminal interbody procedures when combined with supplemental posterior instrumentation.
T‑PAL PEEK Cage
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
(插入产品图片-组图形式)
(插入产品规格的列表)
CAS No. | |
Category | |
Name | |
Molecular Formula | |
Molecular Weight | |
Chemical Structure | |
Form | |
Melting Point | |
Boiling Point | |
Density | |
Storage Temperature | |
Packaging | |
Contact us for Customized Packaging | |
MSDS document |
(插入产品实拍图,多角度实拍,3-5张,做成组图)
A PEEK-based interbody fusion device designed for TLIF or PLIF procedures. Its polymer core mimics natural bone elasticity, while titanium marker pins ensure imaging visibility. The design facilitates graft containment and alignment with minimal subsidence. It’s suitable for minimally invasive or open posterior approaches.
Studies demonstrate that Ti-PEEK composite cages preserve motion segment stiffness while reducing subsidence compared to solid titanium versions. Though traditional PEEK may limit osseointegration, surface treatment or PEEK-Ti composites help improve early fusion rates. Fusion outcomes of PEEK cages are comparable to titanium but often produce lower imaging artifact and equivalent fusion rate (~79–95%).
The T‑PAL PEEK Cage is a versatile interbody fusion implant combining the mechanical advantage of PEEK with imaging clarity offered by titanium markers. Designed for TLIF/PLIF procedures, it offers low subsidence risk, anatomical versatility, and simplified fusion assessment. CE- and ISO-certified, it aligns with contemporary surgical expectations for safety, flexibility, and outcome monitoring.
Ti‑PEEK implants mitigate PEEK’s hydrophobic limitations by improving osseointegration via titanium-enhanced surfaces, while maintaining radiolucency. Early clinical data shows improved fusion surfaces with Ti‑PEEK composites.
Yes—PEEK’s elastic modulus closer to bone reduces interface stresses and subsidence, while Ti‑PEEK composites maintain leverage benefits with fewer imaging artifacts.
Absolutely—the low profile, kidney-bean design with modular sizing supports minimally invasive transforaminal insertion.
Titanium marker pins within the PEEK cage enhance visualization under X-ray and CT, facilitating graft assessment without obscuring detail.
Yes—OEM/ODM options support custom sizes, lordotic angles, and packaging formats.
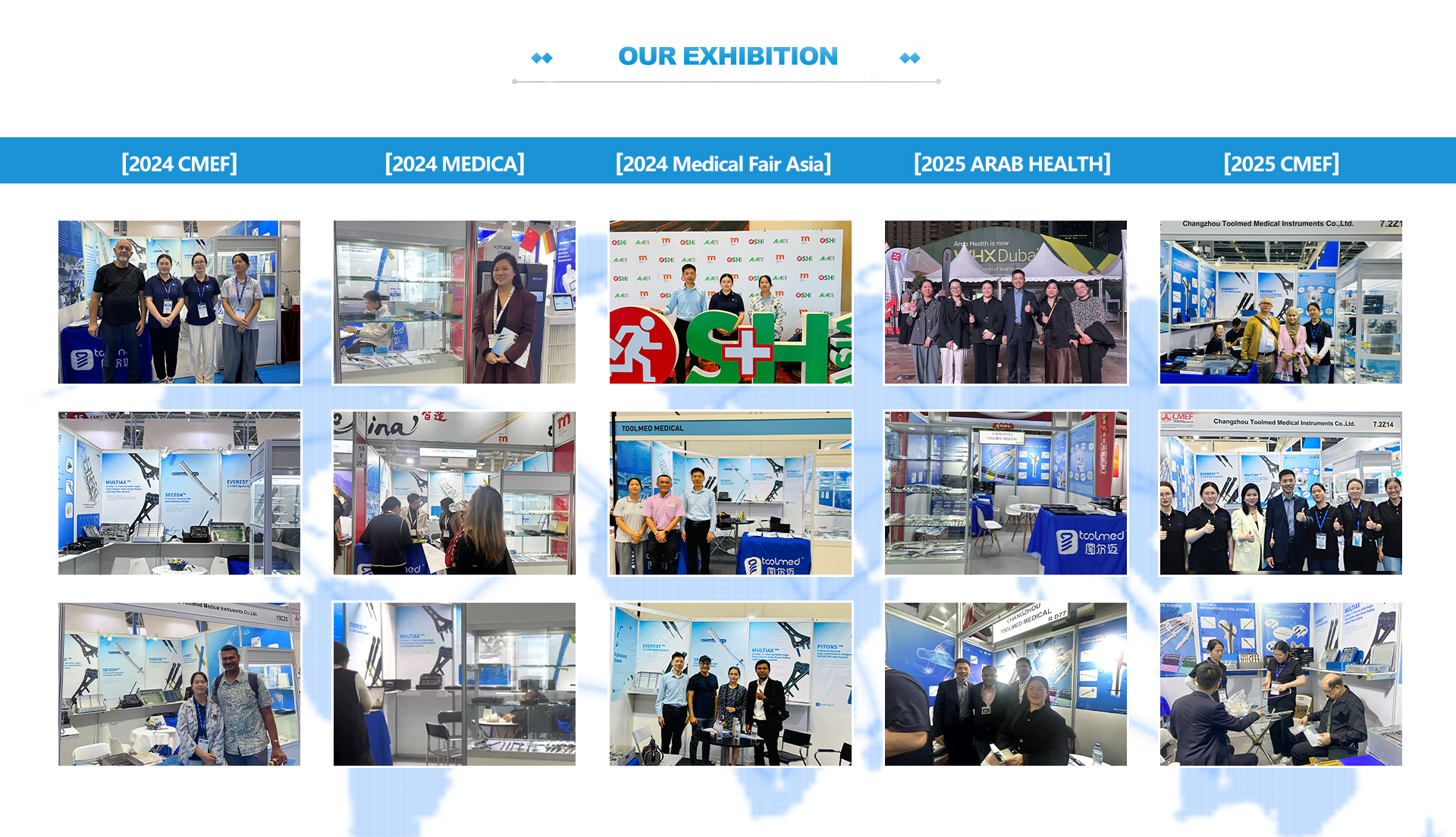
Our factory is equipped with over 60 advanced CNC machines operated by 100+ skilled technicians, a 300 m² GMP-compliant clean room, and a 10,000 m² production facility. We maintain a CNAS and ISO 17025 certified testing center, invest heavily in CNC turning technology, and utilize high-precision inspection systems including CMM, PP, and QV to ensure product quality and consistency.

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us