Spine
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
The PEEK material allows for artifact-free visualization of the fusion process on X-ray and CT scans, enabling surgeons to clearly monitor bone growth through the cage.
The cage is shaped to match the natural curvature of the cervical endplates, ensuring a more uniform distribution of pressure and superior restoration of cervical lordosis.
Strategically placed tantalum pins provide excellent visibility under fluoroscopy, allowing for precise depth and lateral positioning during the surgical procedure.
The serrated teeth on the top and bottom surfaces provide immediate primary stability by gripping the vertebral endplates, significantly reducing the risk of implant expulsion.
Indicated for patients with symptomatic cervical disc protrusions. The cage is used after the damaged disc is removed to maintain the space between the vertebrae and prevent the collapse that leads to further nerve compression.
Used in the treatment of chronic cervical wear and tear. It provides the necessary structural stabilization for segments that have become unstable due to bone spurs or facet joint degeneration in the neck.
Applied to patients suffering from nerve root compression (radiculopathy) or spinal cord compression (myelopathy). By restoring proper disc height, the cage helps decompress the neural structures, alleviating pain, numbness, and weakness in the arms.
The primary solution for cervical DDD (C3-C7). The cage acts as a permanent spacer that facilitates biological fusion at the affected level, eliminating the painful motion associated with a degenerated disc.

| Product name | Cervical Peek Cage-I |
| Material | TA3 |
| Diameter | / |
| Length | / |
| Application | / |
| Certificate | CE Certificate |
| Brand | TOOLMED |
| MOQ | 1 Pcs |
| OEM | Avaliable |
| Package | PE Inner Bag+Carton |
| Payment Method | T/T,Bank transfer, Western Union |
| Delivery Time | 5-7 days |
| Shipping | DHL EMS UPS TNT FEDEX |


The Cervical Peek Cage-I is a specialized interbody fusion device designed for the cervical spine. Manufactured from PEEK-OPTIMA, it serves as a structural replacement for the intervertebral disc after an anterior discectomy. It is engineered to restore anatomical disc height and provide immediate cervical stability, creating a controlled environment that facilitates biological fusion across the disc space.
This cage is the clinical standard for Anterior Cervical Discectomy and Fusion (ACDF) procedures. It is applied to treat radiculopathy or myelopathy resulting from cervical disc herniations and degenerative spondylosis. By maintaining the distracted height of the disc space, the cage ensures the neural foramen remains open, alleviating pressure on compressed nerve roots.
The primary advantage of the TOOLMED "I" series cage is its biomimetic modulus of elasticity, which reduces the peak stresses on the vertebral endplates and minimizes the risk of implant subsidence. Furthermore, its radiolucent properties allow for unobstructed postoperative imaging, enabling surgeons to confirm the presence of a maturing bone bridge without the interference of metallic artifacts.
Biomechanically, the cage functions as a load-sharing spacer that maintains cervical lordosis and sagittal alignment. Its biconvex geometry maximizes the contact area between the implant and the vertebral endplates, while the serrated surfaces provide high frictional stability. This resists shear forces during neck motion, providing the rigid primary stability necessary for osteoconduction.
Using an anterior approach, the surgeon performs a complete discectomy. After preparing the vertebral endplates to bleeding bone, a trial cage is used to select the optimal footprint and height. The Cervical Peek Cage-I is then packed with bone graft, attached to its dedicated inserter, and impacted into the disc space. Final positioning is verified via fluoroscopy using the integrated tantalum markers before supplemental plating if required.
Postoperative care typically includes a short period of neck immobilization using a cervical collar to protect the fusion site. Patients are monitored via regular radiographic assessments to track the progression of the bone fusion. Because PEEK is MRI-compatible, high-resolution follow-up imaging can be performed safely to monitor the neural structures without implant-related distortion.
The TOOLMED Cervical Peek Cage-I provides a reliable and anatomically sound solution for anterior cervical reconstruction. Its high-performance material and optimized design ensure long-term mechanical stability and successful biological fusion for patients suffering from cervical spine disorders.
Yes, the Cervical Peek Cage-I is suitable for both single and multi-level anterior cervical fusions, providing consistent height restoration across all treated levels.
While the cage provides primary stability, an anterior cervical plate is often used as supplemental fixation to provide additional security and higher fusion rates, especially in multi-level cases.
The cage contains tantalum markers that show its exact position on X-ray. The radiolucency of the PEEK itself is a benefit, as it allows doctors to see the bone graft growing directly through the center of the cage.
The biconvex shape matches the natural anatomical curvature (lordosis) of the cervical vertebrae, ensuring a better fit and more even load distribution compared to flat-surfaced cages.
Yes. PEEK-OPTIMA is a highly biocompatible polymer and is an ideal alternative for patients with known sensitivities to nickel or other metal alloys commonly found in titanium implants.
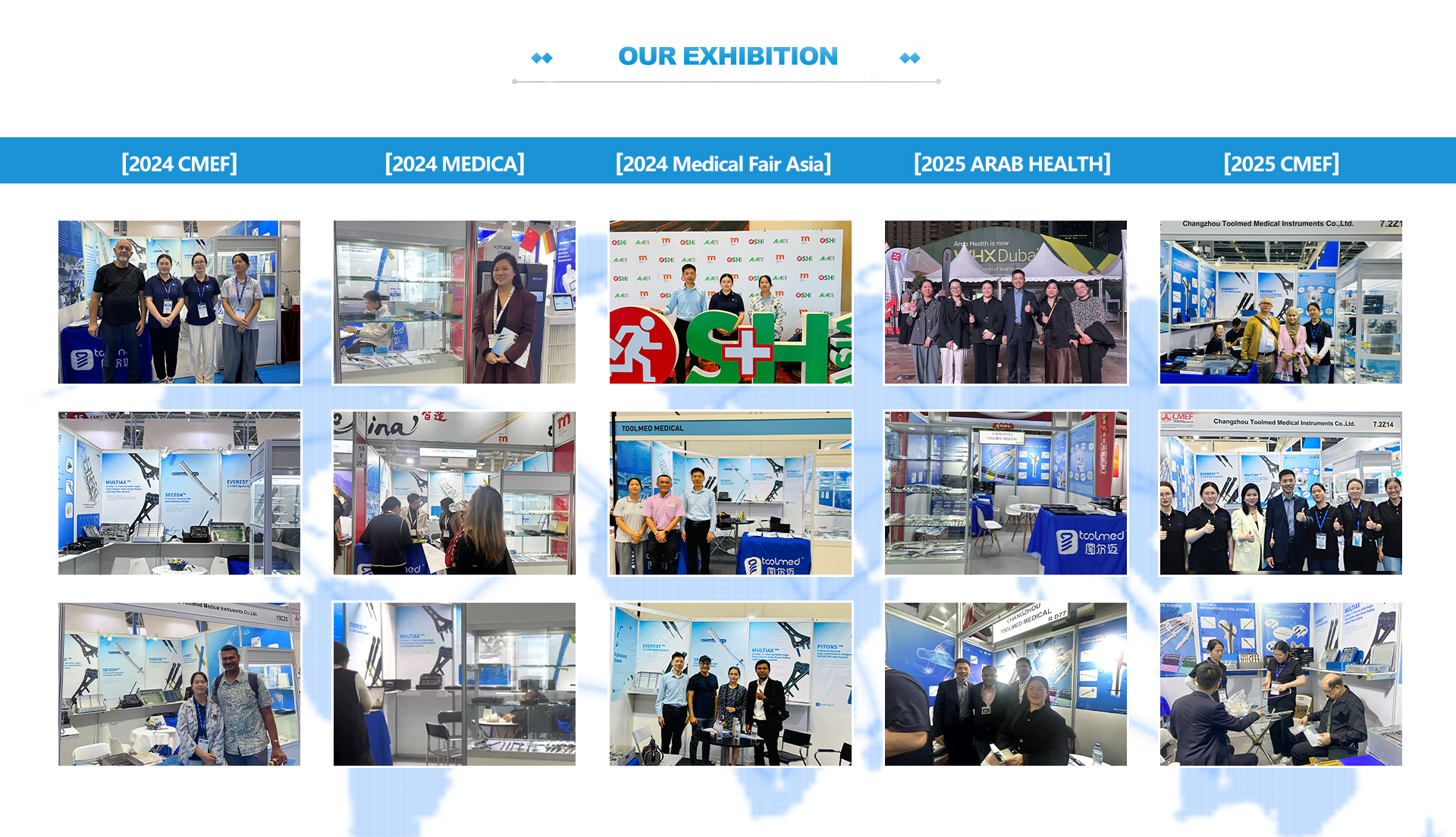

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us