The TOOLMED PLIF PEEK Lumbar Cage & Titanium Lumbar Cage Instrument Set is a comprehensive surgical solution engineered for Posterior Lumbar Interbody Fusion (PLIF). This dual-compatibility set is designed to facilitate the preparation and implantation of both radiolucent PEEK and high-strength Titanium spacers. The kit includes a robust array of anatomical trials, specialized disc space distractors, and universal cage inserters that provide a secure grip on various implant geometries. Key features include ergonomically balanced impactors with vibration-dampening handles and a series of endplate rasps designed to maximize the fusion surface area. Manufactured from medical-grade 17-4PH stainless steel, these instruments are built for high-torque applications and repeated sterilization cycles. The set is housed in a logically organized, multi-layered tray that streamlines the surgical workflow from initial neural decompression to final interbody stabilization. Whether performing a single-level fusion or complex multi-level reconstruction, the TOOLMED PLIF set provides the tactile feedback and mechanical precision required for successful posterior column restoration.
Spine
TOOLMED
/
/
/
1 Pcs
5-7 days
| Availability: | |
|---|---|
Product Description
The set features specialized inserter tips and impactors designed to safely handle both PEEK and Titanium cages, preventing surface marring or material transfer during implantation.
Includes a series of graduated distractors that allow the surgeon to gradually and safely restore disc height, providing the necessary clearance for cage insertion without damaging the vertebral endplates.
The cage inserters utilize a reinforced threading mechanism that ensures the implant remains perfectly axial during the forceful impaction required in posterior lumbar approaches.
Features a variety of curved and straight rasps that allow for meticulous removal of the cartilaginous endplate while preserving the subchondral bone for optimal cage support.
Indicated for the stabilization of high lumbar segments where the spinal canal is narrower. The set provides the precision tools needed to work safely around the conus medullaris and upper cauda equina.
The primary indication for treating degenerative spondylolisthesis and disc herniation. The instruments are optimized for the larger disc spaces and higher lordotic angles of the lumbosacral junction.
Used in the stabilization of traumatic burst fractures where posterior column support is required. The robust impactors allow for the placement of cages into compromised segments to restore height.
Applied in revision cases where previous fusion has failed. The extensive range of trials and specialized curettes allow for the removal of old scar tissue and the precise fitting of a new interbody spacer.

| Product name | PLIF PEEK Lumbar Cage & Titanium Lumbar Cage Instrument Se |
| Material | TA3 |
| Diameter | / |
| Length | / |
| Application | / |
| Certificate | CE Certificate |
| Brand | TOOLMED |
| MOQ | 1 Pcs |
| OEM | Avaliable |
| Package | PE Inner Bag+Carton |
| Payment Method | T/T,Bank transfer, Western Union |
| Delivery Time | 5-7 days |
| Shipping | DHL EMS UPS TNT FEDEX |


The PLIF PEEK Lumbar Cage & Titanium Lumbar Cage Instrument Set is a versatile surgical toolkit designed to facilitate the implantation of interbody spacers through a posterior approach. This set is unique because it is engineered to be compatible with both PEEK (Polyetheretherketone) and Titanium cages. It contains all the necessary preparatory tools—such as trials to measure the disc space height, distractors to open the collapsed space, and inserters to place the permanent cage. By housing tools for both material types in one set, it provides surgical teams with maximum flexibility based on the patient's bone quality and the surgeon's preference for either radiolucent (PEEK) or osteoconductive (Titanium) materials.
This instrument set is applied in Posterior Lumbar Interbody Fusion (PLIF) surgeries, where the goal is to stabilize the spine and promote bone growth between two vertebral bodies. The set is used after a laminectomy or facetectomy to access the disc space. Surgeons use the tools to remove the degenerative disc, prepare the bone surfaces, and then impact two cages (one on each side of the spinal canal) to restore the natural height of the disc space. This application is foundational for treating chronic low back pain, spondylolisthesis, and spinal stenosis where sagittal balance restoration is required.
The primary advantage of the TOOLMED PLIF set is its superior mechanical reliability. The inserters are designed with a high-strength locking mechanism that prevents the cage from "flipping" or detaching during the high-force impaction required in the lumbar spine. Additionally, the trials are manufactured with a specialized "bullet-tip" geometry that facilitates easy entry into collapsed disc spaces. The use of medical-grade 17-4PH stainless steel ensures that the instruments maintain their structural integrity and resistance to corrosion, even after hundreds of autoclave cycles, providing a durable solution for high-volume orthopedic centers.
Biomechanically, these instruments are designed to ensure an optimal "press-fit" construct. The trials allow for precise height selection that tension the annulus fibrosus and ligaments, which provides immediate stability through "ligamentotaxis." The endplate preparation tools are designed to remove cartilaginous barriers without compromising the structural cortical bone of the vertebral endplate. This balance is critical because it maximizes the surface area for bone graft contact while preventing the cage from sinking into the vertebrae (subsidence), ensuring long-term maintenance of the corrected spinal height.
The surgical technique begins with a posterior midline incision and decompression of the neural elements. The disc space is identified and distracted using the set's graduated distractors. The trials are then used to determine the footprint and height of the required cage. After the endplates are prepared using the rasps, the PEEK or Titanium cage is filled with bone graft and attached to the inserter. The surgeon impacts the cage into the disc space under fluoroscopic control. The process is repeated on the contralateral side, and the construct is finally stabilized with posterior pedicle screws.
Postoperative care refers to the rigorous cleaning and sterilization of the instrument kit. After use, each instrument must be manually scrubbed with enzymatic cleaner to remove bone and tissue debris from the serrated tips of the rasps and the threads of the inserters. Ultrasonic cleaning is recommended to ensure all biological material is removed from the hinged joints of the distractors. The instruments are then lubricated and steam-sterilized at 134°C. Proper maintenance of the inserter threads is essential to ensure they continue to provide a secure grip on the cages during future procedures.
The TOOLMED PLIF PEEK Lumbar Cage & Titanium Lumbar Cage Instrument Set is a versatile and robust platform for posterior spinal fusion. By combining precision preparation tools with dual-material compatibility, it empowers surgeons to achieve reliable height restoration and stable interbody fusion in the lumbar spine.
While specifically optimized for the straight posterior approach of PLIF, many of the preparatory tools and trials can be used in TLIF. However, we recommend our dedicated TLIF set for the specific angled inserters required for that approach.
Yes, all height trials are color-coded and laser-marked to match the corresponding PEEK or Titanium cages, making it easy for the scrub technician to provide the correct size during surgery.
The inserter tips are designed with a smooth, large-surface contact area that distributes the impaction force across the cage body, preventing any stress concentrations that could mar the PEEK material.
The standard set includes distractors up to 15mm. If larger distraction is required for high-grade spondylolisthesis, custom-sized distractors can be provided upon request.
Yes, the set features ergonomic medical-grade silicone handles that provide a superior grip even when wet and help to dampen the vibration felt by the surgeon during impaction.
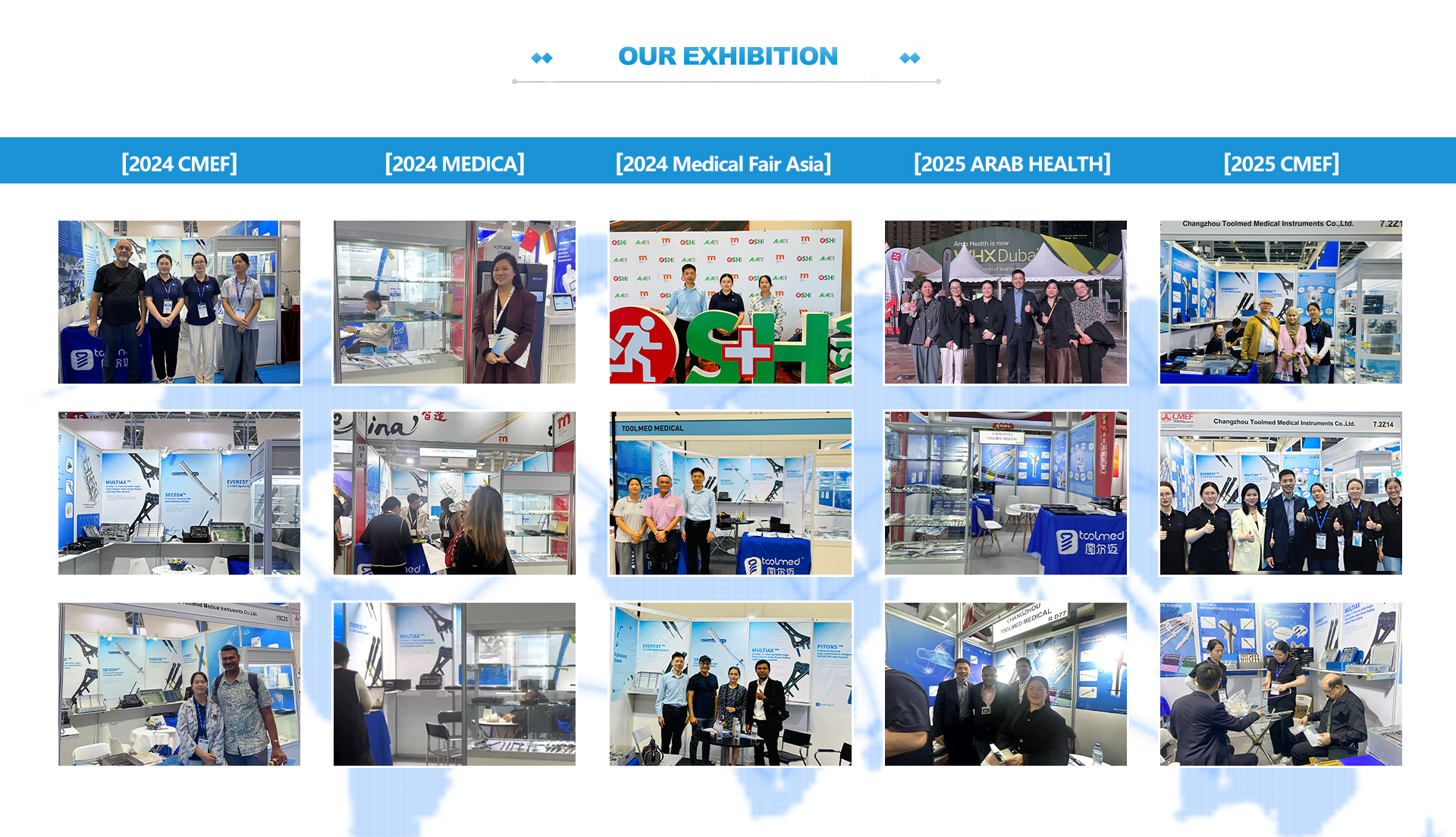

We offer fast and reliable global shipping. Standard lead times range from 7 to 15 working days, depending on order quantity and customization requirements. Express delivery options are also available upon request.
Yes, we ship to most countries worldwide. We work with trusted logistics partners to ensure timely and secure delivery.
We accept a variety of payment options, including T/T (bank transfer), L/C, and PayPal for smaller orders. Payment terms can be discussed based on order volume and partnership agreements.
Yes, samples are available for evaluation. Please contact our sales team to request product samples and shipping arrangements.
Absolutely. We offer OEM/ODM services including custom packaging, branding, and product development tailored to your specifications.
Links
Contact Us