Views: 26 Author: Site Editor Publish Time: 2025-11-28 Origin: Site
Selecting the right orthopedic implant manufacturer is one of the most critical decisions for global distributors, hospitals, and procurement managers. A reliable supplier influences not only product quality and patient safety but also long-term business stability and brand reputation.
Today’s highly regulated orthopedic market demands manufacturers with proven capability, strict quality management, and international service awareness. This guide explains the key evaluation criteria and how a manufacturer like TOOLMED, with 12+ years of industry experience, can meet the expectations of global distributors—without exaggeration, focusing on real and verifiable strengths.
A trustworthy orthopedic supplier must demonstrate strong in-house manufacturing capabilities. This is the foundation of product stability, supply reliability, and long-term partnership.

TOOLMED operates a 10,000㎡+ manufacturing plant, equipped with 60+ advanced CNC machines sourced from Germany, Japan, and the U.S. This allows consistent machining accuracy across plates, nails, screws, and surgical instruments.
The company also maintains a 300㎡ cleanroom and standardized inspection workflow—helpful in ensuring every batch meets international standards.
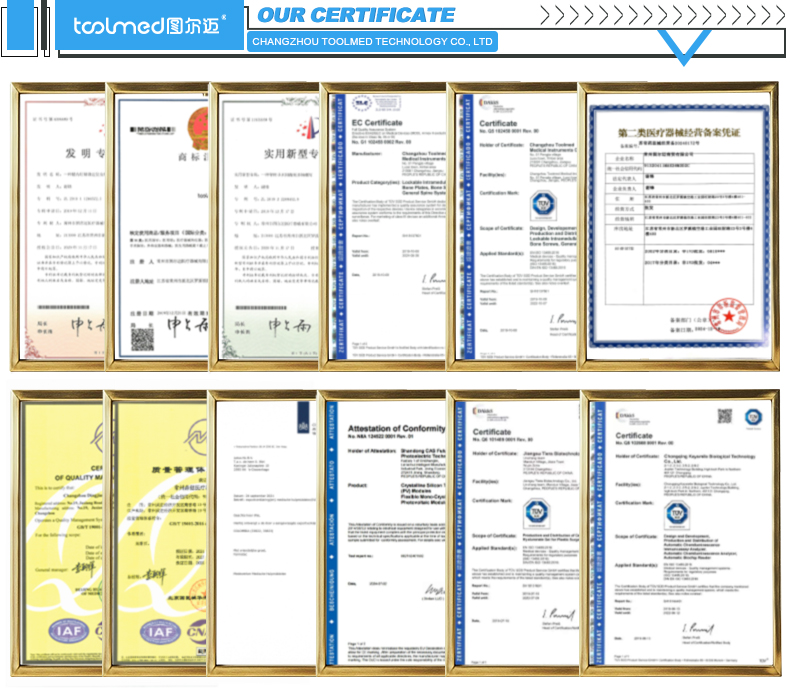
Orthopedic implants are strictly regulated worldwide. Before choosing a supplier, confirm that they comply with globally recognized standards.
TOOLMED Reality
TOOLMED holds CE, ISO 13485, ISO 9001, and GMP certifications—ensuring products meet strict occupational safety, consistency, and regulatory requirements. This also simplifies import approval processes for distributors in Europe, Southeast Asia, Middle East, and Latin America.
A good orthopedic implant manufacturer should provide a wide yet professionally structured product range. This helps distributors grow multi-segment businesses without relying on multiple suppliers.

TOOLMED provides an extensive portfolio covering most major orthopedic specialties. For distributors, this means:
Many markets require custom models, branding, or compatibility with local systems. Strong OEM/ODM capability is a major advantage.

TOOLMED offers structured OEM/ODM services, including:
This is particularly valuable for new distributors entering the orthopedic market.
QC is more than checking finished products—it includes every stage from raw material selection to final packaging.

TOOLMED uses standardized inspection checkpoints, supported by testing devices such as:
These procedures ensure that plates, screws, nails, and instruments maintain reliable mechanical performance in surgery.
For distributors, long lead times can result in lost opportunities. Managing stock and planning surgeries requires a supplier with flexible and quick delivery.

TOOLMED maintains a 500㎡ inventory warehouse, allowing certain products to be shipped in as fast as 1 week.
This responsiveness is particularly helpful for:
Professional manufacturers should be willing to provide samples for verification, especially for long-term partnerships.



TOOLMED offers free samples (with customer paying shipping), which allows distributors to inspect actual product quality before placing larger orders.
Manufacturer credibility grows with real customer experience and market distribution.

TOOLMED’s products have been delivered to 20+ countries, with positive reviews from surgeons, distributors, and hospital buyers. While the company doesn’t overstate its market scale, these verified cases strengthen trust.
Orthopedic implants require responsible and transparent after-sales support.
TOOLMED provides up to 5 years warranty on implants and offers responsive customer service. This reduces risk for distributors expanding into new regions.
Evaluating an orthopedic implant manufacturer requires a systematic approach combining manufacturing capability, certification, quality control, service support, customization, and global presence. A strong supplier does not rely on marketing claims—it demonstrates value through transparent information, measurable performance, and consistent results.
TOOLMED, with its established manufacturing foundation and practical global service experience, provides a solid and realistic option for distributors seeking long-term cooperation. Reliable products, stable delivery, and professional OEM/ODM capabilities make it a trustworthy partner in today’s competitive orthopedic market.
Proximal Radius Locking Plate: Precision Fixation for a Demanding Anatomical Region
The Science Behind Surface Treatment: How TOOLMED Improves Implant Longevity
Why CNC Precision Matters: How TOOLMED Ensures Consistent Quality in Orthopedic Implants
How to Evaluate an Orthopedic Implant Manufacturer: A Complete Guide for Global Distributors
The Rise of Locking Plate Technology: Clinical Benefits and Manufacturing Insights
Links
Contact Us